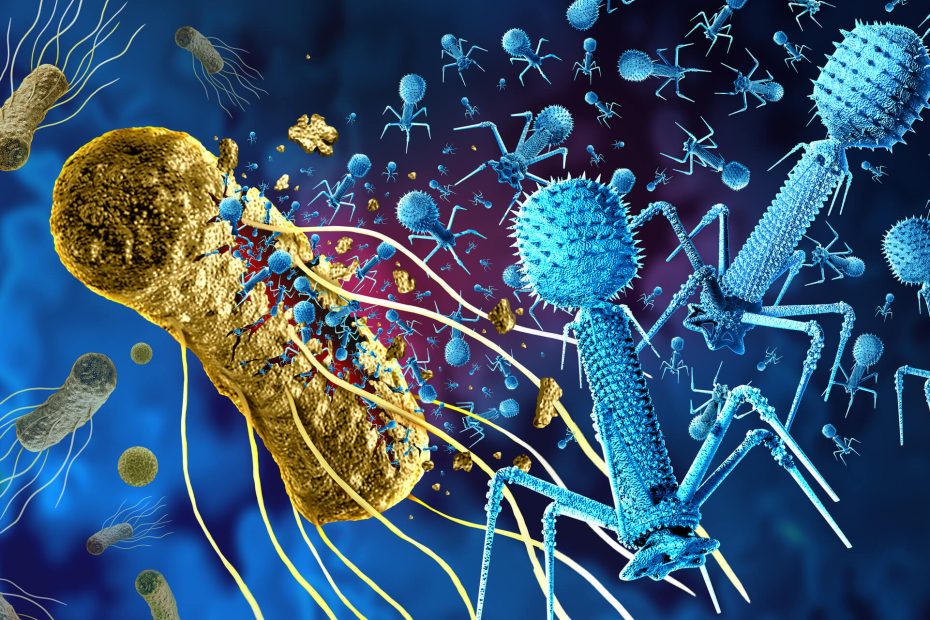
Long before COVID-19, a serious pandemic had been lurking inside hospitals and health care centers worldwide multidrug-resistant (MDR) microbial infections, which are caused by bacterial strains with various mechanisms to resist antibiotics. An increase in antibiotic control and improved health care practices have helped decrease the spread of these bacteria. Still, new bacterial strains and different mechanisms for antibiotic resistance are constantly being discovered, underscoring the rapid rate of bacterial evolution. This constant fight that health care professionals have against bacteria is taking us to new fields for solutions. Currently, research against these MDR bacteria has two main fronts: the use of artificial intelligence (AI) to synthesize new antibiotics and using our yet unknown allies — bacteriophages (or phages).
Bacteriophages are viruses that use bacteria as replication hosts, infecting and eventually killing the bacterial target. Phages exist side by side with their hosts, giving them an advantage over antibiotics: the ability to evolve with their hosts. In nature, bacteria and bacteriophages compete in a race for survival, evolving and counter-evolving to outlast each other. As a defense mechanism against phages, bacteria evolved the well-known CRISPR (clustered regularly interspaced short palindromc repeats) system we use now for biotechnology and medical purposes. However, some phages have developed mechanisms to jump over this wall and infect their hosts. At the same time, bacteria evolve new systems to resist phages. This balance between bacteria and bacteriophage evolution is why researchers are looking into phages to fight MDR bacteria.
The advantages of the phages are clear. First, these viruses are specific to bacteria. Second, after infecting MDR bacteria, they replicate and attack the remaining targets, eliminating the infection entirely. Third, phages are relatively easy to isolate from an infected human host, meaning they can be individualized for each person and bacteria if needed.
Despite these advantages, using phages for antimicrobial treatment is not straightforward. When bacteriophages are isolated and identified, they must fulfill many requirements to be effective therapeutics:
- They should eliminate their host faster than the host can reproduce itself.
- Phages should be lytic, meaning they kill the host instead of becoming integrated into the host genome.
- Depending on the target host, a wide or narrow host range (i.e., the set of bacteria that are susceptible to the page) might be needed.
- The phage should not carry toxic genes that could cause additional harm.
Generally, bacteriophages can be isolated from any environmental sample such as water, human skin or even saliva. Then, phages are characterized and tested for efficacy and safety. For example, researchers will identify what other hosts the bacteriophage targeted to determine if normal bacteria, such as those that comprise the microbiome, are affected.
Unfortunately, this process requires time that, in some cases, a patient does not have. To overcome this limitation, it has been shown that premade bacteriophage cocktails are stable and, like antibiotics, they can help clear infections. Studies are showing how effective bacteriophages can be against MDR bacteria in patients.
In a small trial, patients with diabetic foot ulcers showed improvement after using a commercial staphylococcal phage Sb-1. These patients were reported to have MDR infections that healed after being treated with the phage for several weeks.
In another study, patients with chronic infected wounds were treated with an isolated bacteriophage cocktail. At the end of a two-week treatment, the patients’ wounds were cleared of bacteria, and three months later, nearly half of the wounds had healed completely.
Despite some limitations, bacteriophage therapy promises to be a useful tool to overcome MDR infections in health care facilities worldwide. These small organisms have been shown to be host specific, easy to isolate and safe for patients, and they are built to combat bacterial evolution.
Related Content
- Bugs Resistant to Drugs: What Can We Do?
- Everyday Antibiotics May Reveal New Therapies to Treat Your Breast Cancer
- Antimicrobial Stewardship: Ensuring a Future for Human Health Care
Want to read more from the Johns Hopkins School of Medicine? Subscribe to the Biomedical Odyssey blog and receive new posts directly in your inbox.