If you were diagnosed with a disease, there are two questions you would immediately want answered:
1. How can we treat it?
2. What caused the disease?
Those two questions are the foundation of my Ph.D. thesis. I study a muscle disease called inclusion body myositis (IBM), which is sometimes referred to as the “Alzheimer’s of the muscle” due to the many similarities between the two diseases: They affect the elderly, the diseases slowly worsen over time and, on a cellular level, both have abnormal protein clusters called aggregates. In IBM, these aggregates are called inclusion bodies, which is how IBM gets part of its name. The “myositis” in “IBM” describes the other main feature of the disease, which is the inflammation found in patients’ muscle. Currently, we do not know what causes IBM, and there are no clinically effective treatments. The field needs better laboratory models of IBM, and one goal of my Ph.D. thesis is to address this need.
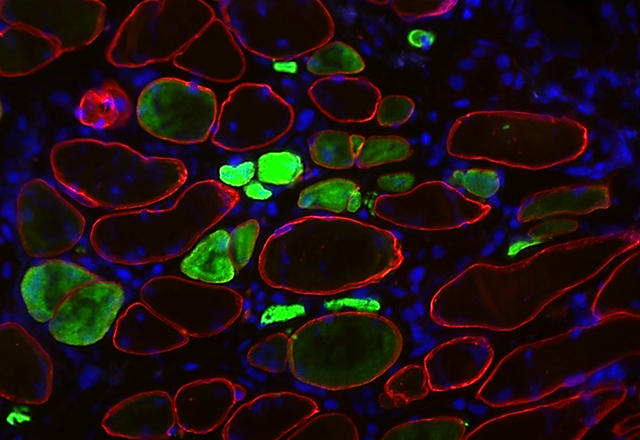
I am developing a hybrid, mouse-human model of IBM called a xenograft model. I receive muscle from a patient with IBM and then graft it into the leg of an immunocompromised mouse host. As this host mouse lacks an immune system, the muscle stem cells are able to regenerate within the mouse to form a functioning human muscle called a xenograft. The image above shows human muscle cells from an IBM patient that have successfully regenerated within a mouse host. The muscle fibers are labeled with three fluorescent markers: spectrin (red) marks the muscle cell plasma membrane, DAPI (blue) shows DNA in nuclei and embryonic myosin (green) shows fibers that are actively regenerating. The intensity of the embryonic myosin staining relates to the development of the fiber: bright green indicates active regeneration, whereas fibers lacking the green staining are more mature.
Remarkably, these IBM xenografts share features of the human disease, including inflammation and protein aggregates. We hope this model will help us better understand the cause of IBM and provide a platform to test potential therapies for the disease.
Britson’s work is featured on our new JHM.Fundamentals Instagram account, which features basic science images from students and faculty members at Johns Hopkins. Do you have cool images to share? Submit them here!