Mundane mistakes can have catastrophic consequences. Ask Archduke Franz Ferdinand’s driver. One wrong turn down a Sarajevo road put the unfortunate archduke face-to-face with his assassin, leading to his killing and igniting World War I. Or for a more contemporary example, talk to NASA. A unit error, with one team using metric and another using imperial, caused NASA to lose contact with their 125 million dollar Mars orbiter.
As a frequent mistake-maker, I too have borne the considerable fallout from my many errors. To be clear, I am being dramatic. I haven’t accidentally triggered one of the most gruesome wars in world history, or lost a cutting-edge spacecraft (yet). But nonetheless, on one fateful June evening, my seemingly small slip-up put me in a world of hurt.
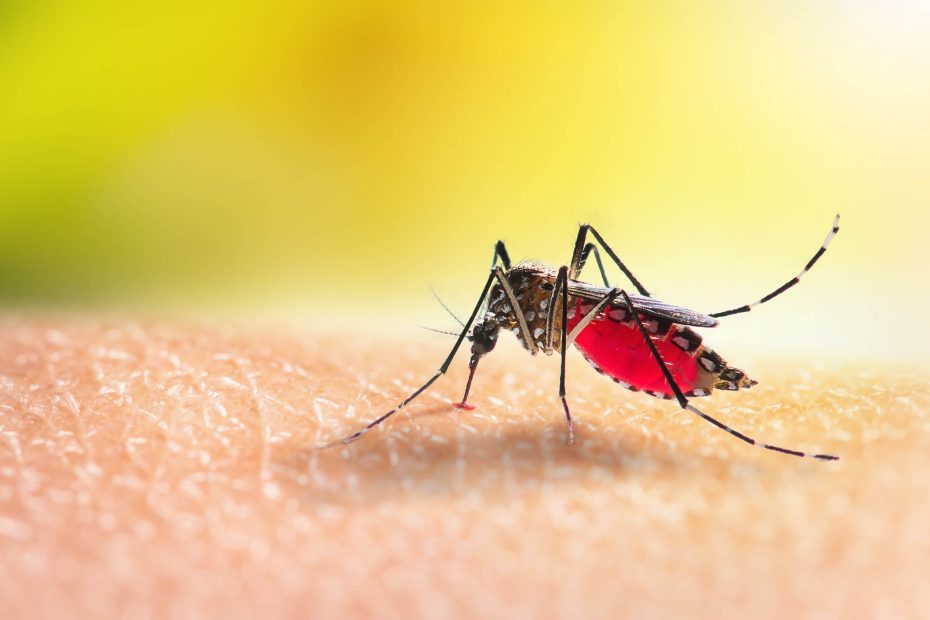
The slip-up began with an observation. After a sweltering Baltimore summer day, largely spent indoors with AC, I saw the sun setting through my apartment window and figured it would be nice to appreciate said sunset from my apartment balcony. It was a seemingly innocuous decision. But little did I know, I had just put my vulnerable body on a collision course with some ravenous mosquitoes.
Mosquitoes possess an uncanny ability to locate their next human meal, somewhat surprising given that they do not possess hawk-like vision. Instead, they use a battery of other sensory modalities. For one, mosquitoes possess cpA neurons that detect carbon dioxide from exhaled breath. They’re also capable of identifying and honing in on the odor of human skin, and possess humidity sensors that alert them to the slightly moistened air that naturally emanates from human skin. They also have finely-tuned temperature sensors that can identify the convective heat rising off of human skin, as well as the thermal infrared radiation emitted from human bodies from a distance of up to 2.5 feet.
As I sat on the balcony, laid-back and chill, those aforementioned ravenous mosquitoes employed all of their tools to find me and burrow their proboscis into my ankle. Evidenced by the half-dozen welts I found on said ankle once I returned inside my humble abode, they had a proper feast. I chastised myself for not applying bug spray, but then laughed at that suggestion. I was on my balcony for goodness’s sakes, not out hiking in a forest. The nearest plant, in a comically small pot, was easily a dozen yards away on a neighbor’s balcony. But, favorable environment or not, the mosquitoes had gotten to me nonetheless.
The next morning, I awoke to a nasty surprise. My bitten ankle – usually bony, sometimes stiff, and always weak, was so swollen it looked like a navel orange somehow got lodged inside it. As I got out of bed and got to my feet, my ankle protested and delivered a sharp pain that caused me to audibly gasp. It felt warm and itchy, and upon further examination, was a concerning shade of reddish-pink. In short, it was infected. Thankfully, I was treated and suffered no additional complications. But as I always am wont to do, my personal experience informed the rabbit holes I (all too frequently) dive into. This incident didn’t feel isolated. From my anecdotal (and therefore nearly worthless) experience, it seemed as if the mosquitoes were getting worse. Was it just me?
Vector-borne diseases, or diseases transmitted by insects including mosquitoes and ticks, exist as a complex interplay between vector, pathogen, and host. In order to spread, all three must be present. For example, for malaria transmission to occur, a female Anopheles mosquito must be present, infected by the malaria parasite, and be able to bite a vertebrate host. That vertebrate host serves as a key link in the chain, as additional mosquitoes must bite that infected host in order to get infected and spread the pathogen further. And many different species of mosquitoes transmit a variety of different diseases. Aedes mosquitoes, a serious global public health threat, transmit any number of diseases from dengue fever to West Nile virus to Zika virus.
I want to be clear though, there are numerous complexities and nuances in the spread of various vector-borne diseases, and what I’ve provided above is an oversimplification. But it’s an instructive one. Nuances or not, what’s clear is that vector-borne disease transmission is complex and dynamic, with multiple steps and players, and is constantly in flux. That inherent volatility is only amplified in the backdrop of climate change and human activity. In a 2024 Nature Reviews Microbiology article, Souza et al. covers the effects of climate change and human activity on vector-borne diseases. The authors are careful to assert that the picture isn’t entirely negative – climate change and human activity may have neutral or even negative effects on specific vectors and diseases. However, their ultimate conclusion is largely unequivocal: climate change and human activity will “undoubtedly reshape the risk and burden of vector-borne disease worldwide in the coming years and decades.” And as we will explore here, profound change, even if net neutral, can have significant, adverse consequences.
Perhaps the most important first step in any article on vector-borne disease, especially one written in the United States, is to make clear their enormous human cost. There are an estimated 300 million cases of vector borne diseases each year, ranging from dengue fever to malaria, worldwide, resulting in over 700,000 annual deaths. I start here because I believe that many Americans largely dismiss the risks that vectors like mosquitoes and their diseases pose. Either due to ignorance, a belief that these vectors are a problem confined to somewhere else, or a confidence that treatments exist here, mosquito bites are more an annoyance than a genuine concern. Apart from being selfish – the sheer scope of the human suffering caused by these diseases makes them worthy of consideration, regardless if Americans are not primarily afflicted by them – this ignorance is shortsighted. Malaria was considered endemic in the US until the 1950s and saw significant spread as far north as Cleveland. Thanks to a broad effort including the clearing of wetlands that mosquitoes used as breeding grounds, the use of insecticides and window screens, and improved diagnostics and treatments, malaria is currently considered eliminated from the US. But that doesn’t mean it will always stay that way.
The first, key step in transmission of a vector-borne disease is having a vector in place, and vectors have ideal environments where they like to be. Mosquitoes, like any organism, have a range of temperatures they are capable of tolerating, and within that range, there is an optimal level at which they are best able to survive and reproduce. For me, that temperature is about 73-75 degrees in the temperature-controlled confines of my apartment, but for mosquitoes, their peak ranges anywhere from 77 degrees to 84 degrees in the case of dengue transmitting-mosquitoes. Mosquitoes generally dislike the cold – they can’t function well below 60 degrees Fahrenheit and will be killed by a hard frost, defined as 2 consecutive hours of temperatures below 28 degrees. They also have an upper limit on temperature, somewhere in the mid 90’s.
But it’s more than just temperature that matters, humidity and precipitation also play a key role. Mosquitoes are more active and bite more frequently in humid environments, peaking at a relative humidity of 90%. Further, precipitation generally aids mosquito reproduction. Mosquitoes generally deposit their larvae in standing water, and intense precipitation forms “stagnant water pools” that are ideal environments for their reproduction. Additionally (and unfortunately), mosquitoes also exhibit a “heads I win, tails you lose” dynamic, similar to that of certain waterborne diseases. Droughts are also noted to increase the risk of mosquito borne disease, because the kind of improvised water storage that is widespread and often necessary during a drought is also a fertile breeding ground for mosquitoes.
So in short, mosquitoes like warmer and wetter environments, but also don’t mind drought conditions where humans, storing water in an effort to survive, help mosquitoes survive. Unfortunately, those very climatic conditions are projected to increase thanks to climate change. Global mean temperatures are projected to rise over the 21st century, and the number and duration of hard frosts in many areas is projected to decrease. This warmer air can hold more water and therefore be more humid. As such, precipitation, or the release of said water from the air, is also projected to increase. And in terms of the location of said precipitation, there is high confidence that the contrast between dry and wet regions will only intensify as climate change progresses — meaning wet areas will likely get wetter (floods, extreme precipitation), and dry areas will likely get drier (drought, extreme aridity). Specifically within the United States, projections from the National Institute of Environmental Health Sciences (a division of the NIH), assuming IPCC climate change trajectories, assert that Aedes mosquitoes will encounter a favorable environment across the entirety of the East and West coasts, as well as most of the entire Midwest by 2080, with a period of rapid expansion between now and 2050. And if you were wondering, Aedes mosquitoes have already been found in 40 states and D.C.
However (as almost always), this is a gross oversimplification. Climate projections are extremely complex, not guaranteed to occur, and generally forecast variability from region to region. Further, these trends may also play a protective effect in some regions. In already hot and humid areas such as Southeast Asia and West Africa, where mosquitoes are already prevalent, additional heating may cause temperatures to exceed optimal ranges for mosquitoes and lead to decreased prevalence. And that makes intuitive sense to me. My family hails from Southeast Asia, and when I went to visit, the heat and humidity were already oppressive. Any hotter, and I could easily see how a mosquito would seek cooler shores.
But this is cold comfort, because for one, those very temperature changes in already sweltering regions may render them uninhabitable for both mosquitoes and humans, leading to migration into cooler areas. As covered earlier in this publication, extreme heat is something that us humans must worry about too. And crucially, from a 30,000 foot view, it appears safe to assume that at the very least, regions once too cold or otherwise inhospitable to mosquitoes will soon become more welcoming to them. These two realities force the consideration of a third. Even if on net, mosquito prevalence remains constant, as some regions become too hot for mosquitoes and colder regions warm up into mosquitoes’ optimal temperature zone, those once-cold regions are also likely where humans are going to be moving, too. From my (admittedly untrained eye), that means increased risk.
But vector-borne disease is about more than the vectors – you don’t contract malaria every time you get bitten by a mosquito. The pathogen itself also is crucial. Here, Souza et al. are unequivocal. Throughout history, the movement of humans has been accompanied by vectors and pathogens. Souza et al. note that Aedes mosquitoes themselves were likely introduced to the Americas from Africa during the translatlantic slave trade. In the present, mass global air travel and globalization serve as powerful drivers of pathogen transmission. Vectors like Aedesalready exist on every continent except Antarctica, meaning that it’s not necessary for an infected mosquito to hop on a long-haul flight to trigger long-range transmission. After all, the odds of an infected mosquito surviving a long-haul flight are low (I can barely survive them myself). The likely mechanism is – a traveler to a country where a disease is endemic contracts a vector-borne disease, then returns home and gets bitten by a vector there, infecting said vector. This newly infected vector now can bite someone else and spread the disease to new hosts. This may seem unrelated to climate change, but Souza identifies it as all inter-related. It’s clear that mass air and land travel is a contributor to climate change as well as the spread of vector-borne disease. But more concretely, Souza et al. note that climate change threatens to precipitate large-scale migration and displacement through these powerful mediums of transportation as a result of rising sea levels, extreme weather, disrupted livelihoods, and conflict. As we move, these diseases will move with us.
It’s important to understand that the risk of long-range, vector-borne disease transmission is not siloed off into the future. A CDC report found that during 2004-2016, “nine vector-borne human diseases were reported for the first time” in the United States, and there have already been epidemics of West Nile virus in the United States. Even Dr. Anthony Fauci was recently stricken by West Nile virus, likely contracted when he was outside his home in D.C. But you might ask me, Ethan, okay, these diseases are spreading. But how dangerous are they? Don’t we have treatments?
Let’s talk about West Nile virus, the most common mosquito-borne illness in the continental US and the one that struck Dr. Fauci. First off, the good news. Only around 20% of people infected by West Nile virus (WNV) develop symptoms, which are most commonly a fever, headache, fatigue, body aches, and vomiting. But unfortunately, around 1 in 150 unlucky people develop severe neurological disease, with symptoms including neck stiffness, disorientation, tremors, paralysis, and coma. There are also no vaccines to prevent or treatments to address a WNV infection.
Dr. Fauci was unlucky. In his firsthand account, he started feeling weak, which soon transitioned to severe exhaustion and a high fever. He was admitted to the hospital, where he was delirious and incoherent. He had “never felt so ill” in his life. Tests later came back positive for West Nile virus. He was unable to swing his legs to sit up from his hospital bed or stand up, and experienced serious issues with his cognition. Thankfully, after several days, he was discharged from the hospital and is currently on the mend, but his story is an instructive one. West Nile is a serious disease, and it’s not the only serious mosquito-borne disease. Dengue, with an approximated 96 million symptomatic cases annually, similarly is asymptomatic or mild in most people. However, some individuals, especially if reinfected, experience severe symptoms including high fever, persistent vomiting with blood, and bleeding from the gums and nose. These individuals can also die from dengue. Dengue, like West Nile, currently has no known treatment, but has a vaccine only approved for use in children who were previously infected by dengue. Unfortunately, the manufacturer has chosen to discontinue production in 2026.
I don’t mean to be a fearmonger here. As mentioned previously, most people infected with these diseases experience no or mild symptoms and recover. Odds are, if you are unlucky enough to get one of these diseases, you will be fine. However, that does not justify complacency. First off, that statement I just made, “you will be fine,” is not universal. Older individuals or those with weakened immune systems, are at elevated risk for severe West Nile infections, among others. Further, it’s a simple numbers game. Even if there is limited individual risk from each infection, given that mosquitoes and their bites are widespread in the environment (if you need firsthand evidence, refer to the beginning of this article), the population-level impact is likely to be significant if mosquitoes and mosquito-borne pathogens become more endemic in the United States. A 0.5-1% chance of severe illness sounds low, but with enough infections, the resulting toll of severe illness can be significant. If you roll dice enough times, you will get snake eyes.
This is not a theoretical risk. Puerto Rico, Peru, and 9 of 26 Brazilian states have declared states of emergency over outbreaks of dengue fever in 2024, and as I explained in the beginning of this article, the human cost of these diseases is astronomical. Further, our healthcare systems and pharmaceutical might, while powerful, are no panacea here. While we can, in theory, deliver more comprehensive medical care than what may be available in some other countries, recall that diseases like dengue and West Nile have no currently known treatments. In short – if these diseases become widespread in the United States, we will pay a heavy price.
To review, Souza et al. and other experts agree that climate change and human activity, through self-reinforcing mechanisms, are likely to expand the ranges of mosquitoes and mosquito-borne disease into new, previously unaffected regions. But the impact of this expansion cannot be fully understood without consideration of the last key player in vector-borne disease transmission: hosts. Or in other words, us. Crucially, even if vectors are present and they harbor pathogens, infections require hosts. And unfortunately, at the moment we appear very vulnerable. As mosquitoes and their diseases encroach on new territory, the humans and societies they encounter will have little familiarity with them.
Take the state where this article is published – Maryland. In 2024 so far, there were 51 reported cases of dengue in Maryland, 0 of which were locally acquired. The average Marylander probably has zero experience and little, if any, knowledge of what dengue is, its symptoms, or means of preventing it. Similarly, the average Maryland physician, hospital, and public health department likely has limited experience with treatment for, coordination of care for, and public health responses against dengue. A Google search for “Maryland dengue fever,” filtering for .gov domains, yields a one-paragraph-long page on mosquito-borne diseases from the Maryland Department of Agriculture, with dengue only mentioned as a “lesser known but frequently occurring” illness in the Americas. There’s also a single page about arbovirus (virus transmitted by arthropod vectors) testing for healthcare professionals from the Maryland Department of Health which itself contains no actual information, but instead links to other memos and fact sheets.
Compare that with Brazil, whose people (unfortunately) possess more familiarity with the disease and its symptoms. Hospitals treat hundreds of patients with dengue daily and public health departments run campaigns to encourage use of bug spray, removal of stagnant water, and wearing long sleeves. Fog machines spraying low concentrations of insecticide are deployed to kill mosquitoes. Further, Brazil has launched a mass vaccination effort, using Japan’s Qdenga vaccine, which is not available or approved in the United States, and coordinated responses around a national arbovirus control room. While this effort is far from perfect, and in many respects it has been insufficient, the level of preparedness certainly outstrips what we have in Maryland.
I don’t mean to dunk on Maryland here. The lack of preparation is understandable and logical given that resources are limited. Problems in the present take precedence. In the present, our dengue situation is nothing like Brazil’s, and credit to Maryland, the Department of Agriculture works with the Department of Health to conduct routine and additional mosquito control via spraying of insecticide across the state. But unfortunately, that may not be enough. The CDC assessed the best estimate of the theoretical range of Aedes mosquitoes in 2017, and even then, most of Maryland had an environment rated as “very likely” to foster Aedes survival and reproduction. I have experienced firsthand the heat, humidity, and precipitation here in Baltimore, and it makes intuitive sense that mosquitoes would find fertile ground here. And that’s not even considering the future trajectory of climate change, which, I’ll remind you, forecasts the entire East Coast as being hospitable to Aedes in the coming decades.
I think the comparative between Brazil and Maryland is instructive because if dengue or other mosquito-borne diseases were to increase in incidence in new areas, as Souza et al. and others forecast, these diseases will encounter populations with limited measures already in place to combat them. Extensive work will be needed to prepare these regions for new disease outbreaks. The United States is lucky to have a wealth of financial resources, human capital, and a deeply flawed, but nonetheless impressive healthcare system. I have zero doubt that if mosquito-borne disease were to spread widely within the US, the nation would mount a considerable response, because we have what it takes. However, having versus actually doing what it takes are two very different things. As our bungled response to the COVID-19 pandemic showed, having deep pockets is no silver bullet. The aforementioned flaws in our healthcare system are, to put it mildly, considerable, and our unfamiliarity with these vector-borne diseases poses a genuine obstacle to confronting them. This doesn’t even take into account the plight of low and middle income countries, who may be similarly unfamiliar with these diseases and already experience serious health inequities. Climate change is a global phenomenon, and unfamiliar or not, these changes are likely to come.
This unfamiliarity also extends deeper, into our very immune systems. When mosquito-borne diseases enter new regions and encounter new populations, the people they encounter will likely have little past immunological exposure to these diseases. This only heightens the risks these diseases pose. In the case of malaria, innate and acquired immunity in malaria survivors leads to reduced risk from subsequent infections. That is not to say that these reinfections are harmless, but rather that the risk of any individual reinfection is likely lower than a comparable infection of someone who has never had malaria before. Studies on malaria transmission in Africa note that communities living in highland regions, where malaria is uncommon due to the colder temperatures, can experience comparatively “devastating” outcomes from malaria outbreaks when warmer climatic conditions allow for an increase in malaria incidence in these regions. Importantly, this pattern does not hold true for all mosquito-borne diseases. For example, dengue fever is more dangerous when one is reinfected. But regardless, individual exceptions do not eliminate the broader risk that a lack of population-level immunity poses. As a plethora of new mosquito-borne diseases meets new populations, newly infected individuals may experience worse outcomes from disease than what we see from other populations with greater past exposure to these diseases.
In summary, mosquitoes generally prefer warmer and wetter weather, which climate change threatens to bring to more of the globe. Extreme heat, along with other extreme weather events precipitated by climate change, may drive both humans and mosquitoes from already warm regions of the world to cooler ones, creating waves of human and mosquito migration that will help spread mosquito-borne diseases such as dengue fever and West Nile virus to new regions. These diseases can cause serious illness and death, and often lack treatments and/or vaccines. With enough infections, even if the per-infection risk is low, the cumulative cost in human lives suffering can be enormous. Compounding that potential suffering is the reality that the regions where mosquito-borne diseases are expanding to are largely unprepared, both societally and immunologically, for these diseases. This may worsen the already concerning potential outcomes from future outbreaks of mosquito-borne disease in these regions.
Crucially, all these issues feed on one another if climate change continues. As climate change worsens, more regions will become warm and wet enough to harbor large mosquito populations, increasing the risk for mosquito-borne disease in more areas. Human migration will also likely increase as climate change worsens, further intensifying the risk of disease transmission. Health systems and governments will be put under immense, increasing strain, forced to address multiplying outbreaks in more regions, along with the broader, incalculable stress that climate change and extreme weather will put on them. This strain, along with the disruptions caused by extreme weather itself, may lead to less effective disease and mosquito control, further exacerbating the potential frequency and impact of outbreaks. It’s a bad feedback loop.
The picture I’m painting, without question, is grim. But inasmuch as I am deeply concerned about this situation, potential solutions exist. The first, and most critical step is addressing climate change. Every step I talk about next will largely be ineffectual in the long-term if climate change continues to intensify and the vicious feedback loop I talked about earlier is allowed to continue. Large-scale emissions reduction and a transition away from fossil fuels is necessary. But apart from that broader goal, more targeted options are also critical. Individuals should use insect repellent, wear long sleeves, consider using bed nets, empty standing water, and be aware of mosquito-borne disease symptoms during periods of high mosquito activity. Public health departments should initiate large-scale campaigns to educate individuals about these critical steps. Further, greater population-level surveillance of vector-borne disease incidence is warranted to better characterize spread and improve targeting of vector control strategies. Vector control strategies, like spraying and fogging of insecticide, should also be enhanced and scaled up if local mosquito prevalence warrants it. Further, novel control strategies should be pursued. Brazil is currently working with the World Mosquito Program on a project to create Aedes mosquitoes deliberately infected with Wolbachia bacteria. This controls mosquito-borne diseases in two potential ways. For one, when male Aedes mosquitoes born from eggs infected with Wolbachia mate with wild female Aedes mosquitoes, the resulting mosquito eggs do not hatch. This helps control mosquito populations. Additionally, Wolbachia infection itself helps inhibit dengue and other arbovirus replication in mosquitoes infected with Wolbachia, aiding reductions in disease transmission. Also, female mosquitoes infected with Wolbachia also transmit the Wolbachia infection to their offspring, allowing these potential benefits to self-perpetuate in mosquito populations. Additional research should be done on vector control methods such as Wolbachia, along with novel vaccines and treatments for vector-borne diseases, to help limit the repercussions of vector-borne disease outbreaks.
I must admit that these approaches have risks and drawbacks. Deliberately infecting mosquitoes with Wolbachia invites the potential risk of unintended consequences, especially when these infections can transmit from generation to generation. Further, spraying of insecticide can harm the environment and be toxic to a variety of wildlife. Effective and judicious implementation is critical, and additional research should be done to find ways to reduce deleterious consequences and/or find better alternatives. But in my estimation, the risks posed by inaction outweigh the risks of action. All of this is easier said than done, but when we face a threat like the one I’ve explored here, I believe ambitious, rapid, and strategic action is warranted.
We’re approaching winter here in Baltimore, and thankfully, that means that the risk posed by mosquitoes when I sit on my balcony has fallen precipitously. But when spring and summer invariably return, the mosquitoes will be back, likely in force. I’ve learned my lesson after my mistake – mosquitoes are no joke, and doing nothing about them means I’m gonna get bit. But it remains an open question whether we as a society will make the same mistake I did. Mosquitoes are here. More are coming. The only question that remains is whether we will make the mistake of doing nothing about them.
Related Content
- Persistent Parasite Proteins: How Protein Clearance in Malaria Infection Can Impact Diagnostics
- Visualizing the Salivary Gland of a Mosquito
- The Fight Against the Brain-Eating Amoeba
Want to read more from the Johns Hopkins School of Medicine? Subscribe to the Biomedical Odyssey blog and receive new posts directly in your inbox.