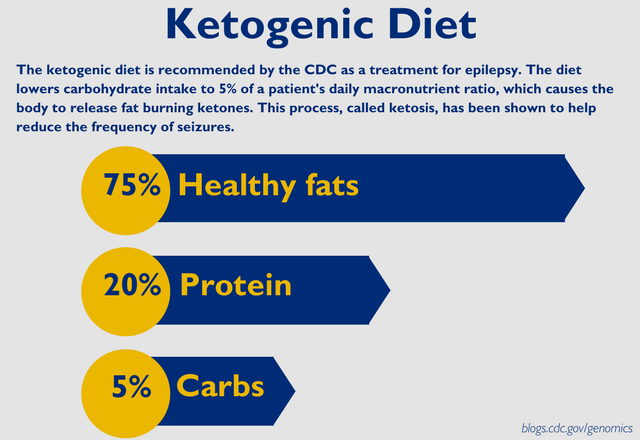
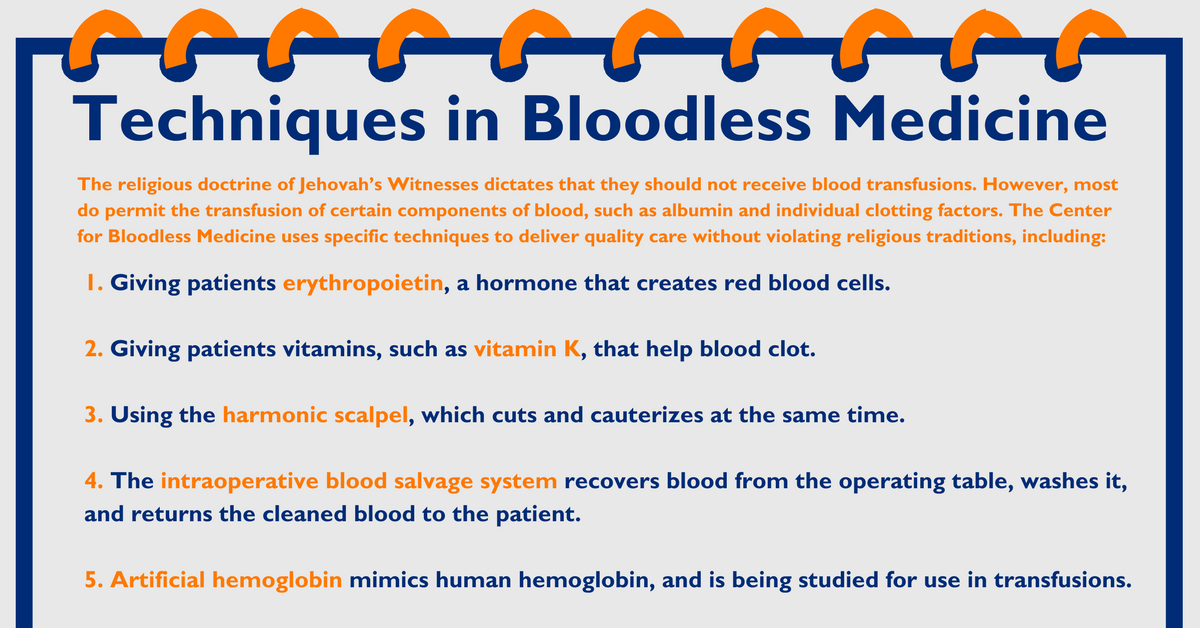
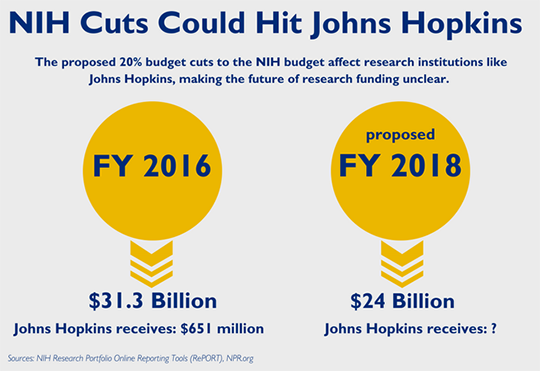
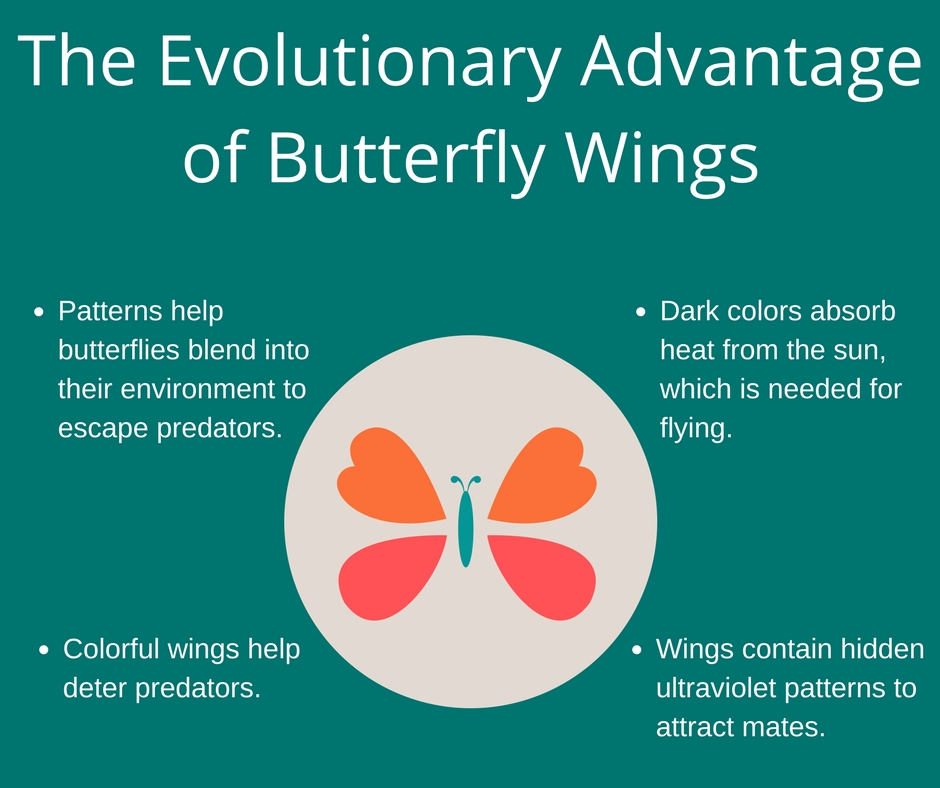
The Impact of Diet on Neurological Disease
Diet—what and how people eat—is becoming a powerful potential intervention for a variety of debilitating neurological diseases. The ketogenic diet, in particular, has been a… Read More »The Impact of Diet on Neurological Disease